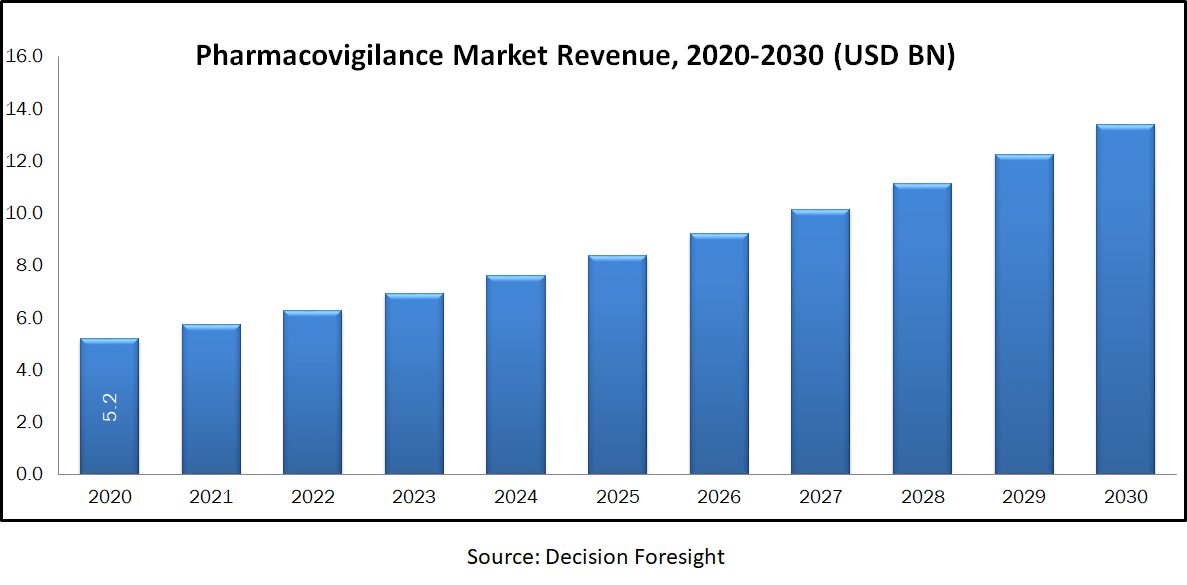
Pharmacovigilance Market size accounted 5.20 billion in 2020 is estimated to reach 13.4 billion by 2030 growing with a CAGR of 10% during the forecast period. Pharmacovigilance is associated with drug safety which includes gathering, detecting, monitoring and preventing adverse reactions of the drug. It mainly focuses on adverse drug reactions & also sides effects of drugs. The information collected from healthcare providers and patients plays an important role to transpire. The major purpose is to minimize the adverse effects of drugs on the patients. It contains preclinical research, clinical research, drug delivery and development, post-marketing surveillance and other medical advancements. This technology used to discover and study every cycle of the drug in pharmacovigilance, from preclinical to post-marketing surveillance.
Market Segmentation:
On the basis of a clinical trial, the pharmacovigilance market is majorly segmented as pre-clinical, phase 1, phase 2, phase 3, and phase 4. By Type, the market is classified into spontaneous reporting, intensified ADR reporting, & targeted spontaneous reporting. By service provider, the market segmented as In-house and contract outsourcing market. On the basis of end-users, the market is categorized into hospitals, research institutes, and industries. By Region wise, the pharmacovigilance market is classified into Asia Pacific, North America, Europe, and RoW.
Market Dynamics and Factors:
An increase in chronic diseases such as oncological diseases, diabetes, and cardiovascular and respiratory disorders has led to an increase in drug consumption worldwide. Thus, the demand for new drug development via extensive clinical trials has increased. Pharmacovigilance is a necessary part of drug discovery and development procedures. Increasing incidence of Adverse Drug Reactions (ADRs) is expected to boost the demand for these services. The presence of a competitive environment for introducing new molecules in the pharmacovigilance market has led to high demand for improved manufacturing operations, pharmacovigilance, clinical research management, streamlined R&D, and medical writing. Manufacturers are rapidly considering outsourcing as a viable cost curbing tool. Expansion helps to increase internal resource flexibility, improve timelines, and gain better outcomes in the short- and long-term. It also helps attain better pharmacovigilance through regulatory compliance, better productivity, higher quality, and improved strategic decisions. Global pharmacovigilance market is driven by the rising awareness about the safety and efficacy of drugs among the worldwide population and the growing demand for substantially adopting safe medical practices. Emerging economies, like India, China, and Brazil, South Africa have gathered momentum due to the increasing government support to open pharmacovigilance centers. This is further driving this global market’s growth. Additionally, the implementation of stringent post-market monitoring mechanism set by various government agencies for effective drug regulation system is also catalyzing the global pharmacovigilance market. The rising prevalence of adverse reactions caused due by drugs has inclined the biotechnological and pharmaceutical industries to adapt pharmacovigilance practices, which, in turn, is also expected to fuel pharmacovigilance market in the future.
Geographic Analysis:
Europe is anticipated to hold a global pharmacovigilance market share in the years ahead, due to the introduction of new laws by the European Union in 2012, which ensures good vigilance practices for medicine regulators and pharmaceutical companies. Rising prevalence of adverse drug reactions and the introduction of the new legislation have augmented the adoption of pharmacovigilance in many companies, which is fueling the European pharmacovigilance market growth. North America is expected to hold the second-largest share of the pharmacovigilance market across the globe, due to the increasing number of research and development activities, technological advancement, substantial developments of new drugs, and flourishing biotechnology and pharmaceutical sectors in the U.S.
Competitive Scenario:
Some key players in the global pharmacovigilance industry include F. Hoffmann-La Roche, Cognizant, Pharmaceutical Clinical Trial Phase Development, Bristol-Myers Squibb, Covance, ICON, Accenture, Pfizer, Clinquest Group, Novartis International, GlaxoSmithKline, PAREXEL International, iGATE Corporation, iMEDGlobal Corporation, and inVentiv Health.
Pharmacovigilance Market Report Scope
| Report Attribute | Details |
| Analysis Period | 2020–2030 |
| Base Year | 2021 |
| Forecast Period | 2022–2030 |
| Market Size Estimation | Billion (USD) |
| Growth Rate (CAGR%) | 10 % |
|
| By Clinical Trial Phase- (Pre-Clinical, Phase I, II, III, IV), By Service Provider (In-House and Contract Outsourcing), By Type (Spontaneous Reporting, Intensified ADR (Adverse Drug Reaction) Reporting, Targeted Spontaneous Reporting, and Cohort Event Monitoring), By End Use (Hospitals, Research Organizations, and Industrial) |
| Geographical Segmentation | North America (U.S., Canada, Mexico) Europe (UK, Germany, Italy, France, Rest of Europe), Asia-Pacific (China, Japan, India, Australia, Rest of APAC), South America (Brazil, Argentina, Rest of SA), MEA (UAE, Saudi Arabia, South Africa) |
| Key Companies Profiled | F. Hoffmann-La Roche, Cognizant, Pharmaceutical Clinical Trial Phase Development, Bristol-Myers Squibb, Covance, ICON, Accenture, Pfizer, Clinquest Group, Novartis International, GlaxoSmithKline, PAREXEL International, iGATE Corporation, iMEDGlobal Corporation, and inVentiv Health. |