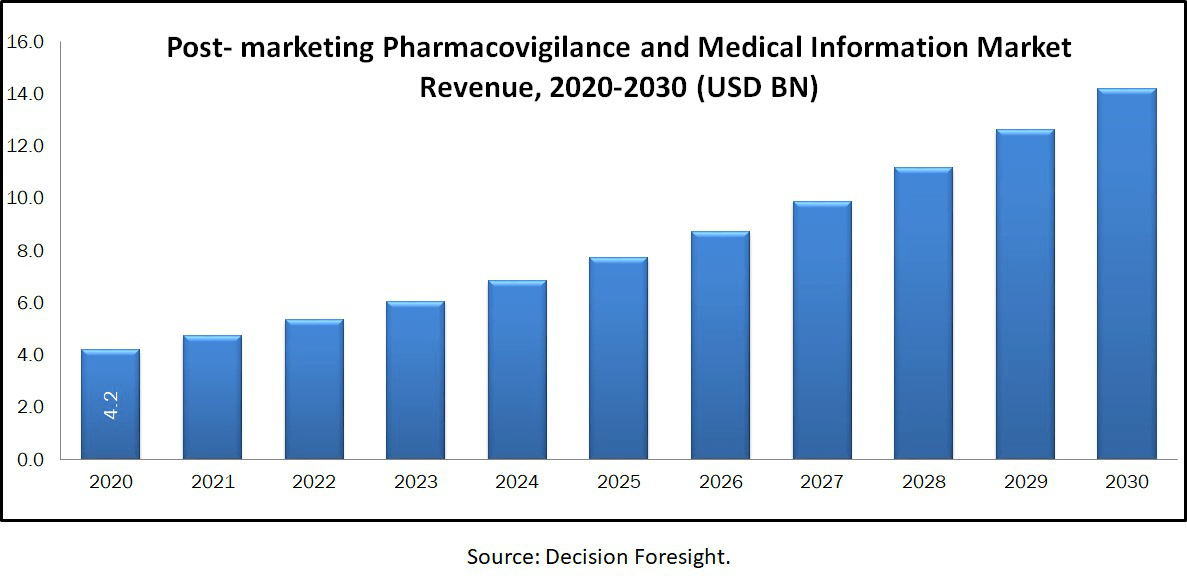
Post-marketing Pharmacovigilance And Medical Information Market size accounted 4.20 billion in 2020 is estimated to reach 14.2 billion by 2030 rowing with a CAGR of 13% during the forecast period. It is the practice of monitoring the safety of a pharmaceutical drug or medical device after it has been released in the market. It is a key part of the science of pharmacovigilance. It is also known as phase IV. Rising demand for new drugs developed with the presence of a technologically advanced, easily accessible medical information system.
Market Segmentation:
On the basis of type, the global post-marketing pharmacovigilance and medical information market is fragmented into spontaneous reporting, intensified adverse drug reaction (ADR) reporting, targeted spontaneous reporting and cohort event monitoring and electronic health record (EHR) mining. On the basis of product, the market is classified into books, online media and journals. By end-users, the market is bifurcated into hospitals and research organizations. And by region, the post-marketing pharmacovigilance and medical information market is divided into North America, Europe, Asia-Pacific, and RoW.
Market Dynamics and Factors:
Drug consumption has increases, due to the rapid growth of disease prevalence rates. In addition, Rise in the impact of chronic diseases such as oncological diseases, diabetes, cardiovascular disorders, respiratory problems has increased the consumption of drugs across the globe. Owing to, these factors the post-marketing pharmacovigilance and medical information market is anticipated growing in the future. Moreover, the increasing prevalence of adverse drug reaction imposes a considerable burden on the healthcare system and is one of the prominent reasons for morbidity. Growing instances of ADRs will positively affect the market in the future. The presence of a competitive environment has led to the streamlining of R&D, manufacturing operations, medical writing, pharmacovigilance, and clinical data management. Cohort event monitoring is anticipated to be the fastest-growing in post-marketing pharmacovigilance and medical information owing to application in all types of clinical events. It is expected to grow with a CAGR of 13.4% during forecast period due to introduction of technologically advanced cloud based platforms and reliability on information obtained by this system. Moreover, associated benefits like early detection, high accuracy, and cost-efficiency are also anticipated to be the growth inducing factors.
Geographic Analysis:
According to research, European Network of Centers of Pharmacoepidemiology and Pharmacovigilance (ENCePp) is expected to show significant growth in the upcoming years due to rising government initiatives incorporating reporting methodology, other than spontaneous reporting. The benefits of targeted spontaneous reporting such as higher affordability, lower labor costs, feasibility in poor resource environments, and routine monitoring should further boost the post-marketing pharmacovigilance and medical information market. Increasingly, electronic health record (EHR) mining is being used to identify patient risk factors after hospital release. North America and Asia-Pacific is expected to grow with maximum CAGR during forecast period.
Competitive Scenario:
The key players operating in the post-marketing pharmacovigilance and medical information industry are Accenture Plc, Clinquest Group, B.V.,Cognizant Technology Solutions Corp., Covance Inc., IMD global Corp., inVentiv Health Clinical, Parexel International Corporation, Pharmaceutical Product Development Inc, Sanofi, PRA Health Sciences, Quintiles Transnational Corporation, F. Hoffmann-La Roche Ltd., Synowledge, Wipro Ltd., ArisGlobal and Ergomed.
Post-marketing Pharmacovigilance and Medical Information Market Report Scope
| Report Attribute | Details |
| Analysis Period | 2020–2030 |
| Base Year | 2021 |
| Forecast Period | 2022–2030 |
| Market Size Estimation | Billion (USD) |
| Growth Rate (CAGR%) | 13 % |
|
| By Type (Spontaneous Reporting, Intensified ADR (Adverse Drug Reaction) Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, EHR (Electronic Health Records) Mining), By Product (Books, Online Media, and Journals), By End-User (Hospitals and Research Organization) |
| Geographical Segmentation | North America (U.S., Canada, Mexico) Europe (UK, Germany, Italy, France, Rest of Europe), Asia-Pacific (China, Japan, India, Australia, Rest of APAC), South America (Brazil, Argentina, Rest of SA), MEA (UAE, Saudi Arabia, South Africa) |
| Key Companies Profiled | Accenture Plc, Clinquest Group, B.V.,Cognizant Technology Solutions Corp., Covance Inc., IMD global Corp., inVentiv Health Clinical, Parexel International Corporation, Pharmaceutical Product Development Inc, Sanofi, PRA Health Sciences, Quintiles Transnational Corporation, F. Hoffmann-La Roche Ltd., Synowledge, Wipro Ltd., ArisGlobal and Ergomed. |